Chimeric antigen receptor-engineered (CAR)-T cell therapy represents one of the most promising strategies of cancer treatment, and the function and persistence of CAR-T cells in vivo dictate the therapeutic success. Understanding the heterogeneity of CAR-T cells at single-cell resolution and deciphering the functional sub-populations and their dynamic changes over time post-infusion that contribute to long-term response or resistance has a crucial implication for the next generation of CAR-T cell therapy.
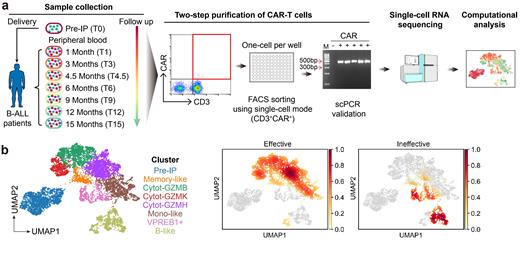
We collected 11 samples of pre-infusion CAR-T products (pre-IP) (dubbed as stage T0) as well as 49 samples of peripheral blood at the peak (T1, the peak of CAR-T cells expansion within one month), early (T3 or T4.5) and late (T6 to T15) stages from 26 B-ALL patients, who received CAR19/22 T-cell cocktail therapy. The functional status of CAR-T cells in vivo at the time of sampling was judged as effective if patients were in B-cell aplasia, or ineffective if patients met the criteria of B-cell recovery. Totally, 36 effective samples and 10 ineffective samples were obtained. Single CAR-T cells with immunophenotype of CD3 +CAR + and expression of CAR sequences were subjected to modified STRT-seq (Figure a).
Cell cycle,cytotoxicity, immune checkpoints, and memory features of CAR-T cells in serial CAR-T cell samples were examined and summarized at the sample level. Pre-IP CAR-T cells were characterized by high cell cycle and low activation status as expected. After infusion, CAR-T cells showed significantly elevated cytotoxicity and declined memory scores, implying leukemia clearance post-infusion. In contrast, during the entire follow-up, cytotoxicity scores sustained high. Interestingly, immune checkpoints scores were positively correlated with cytotoxicity scores in vivo, although stayed relatively low levels in most samples. Specifically, effective samples harbored significantly higher cell cycle, cytotoxicity and immune checkpoints scores at both peak and late stages compared with ineffective samples.
Unsupervised clustering of 7,578 single CAR-T cells resulted in eight molecularly distinct clusters (Figure b). Pre-IP CAR-T cells from different donors, presenting relatively homogenous transcriptomic characteristics, merged into Pre-IP cluster, characterized by highly expression of glycolysis-related genes. Contrarily, CAR-T cells in vivo, displaying evident heterogeneity across patients and stages, were divided into seven clusters. One cluster with noticeable memory characteristics was named Memory-like. Three clusters with clear cytotoxicity characteristics and predominant expression of different granzyme genes were named Cytot-GZMB/GZMH/GZMK, respectively. Among them, Cytot-GZMH exhibited the highest cytotoxicity scores, and Cytot-GZMK had the highest immune checkpoints scores and specifically expressed several inhibitory markers. Notably, three atypical CAR-T clusters were transcriptomically similar to both T lymphoid lineage (CTL/naïve T) and other lineages (Mono/cDC/B/ProB). Accordingly, they were dubbed Mono-like, VPREB1+, and B-like, respectively.
Single CAR-T cells could be roughly tagged as being ‘effective’ or ‘ineffective’ according to the sample-wise functional status. ‘Effective’ CAR-T cells were enriched in three cytotoxic clusters Cytot-GZMB/GZMH/GZMK and cluster Mono-like. Conversely, ‘ineffective’ CAR-T cells were predominantly enriched in two atypical CAR-T clusters, namely B-like and VPREB1+. These results suggested that the clusters Cytot-GZMB/GZMH/GZMK were functional CAR-T cells, and the cells in clusters VPREB1+ and B-like were dysfunctional (Figure b).
Taken together, extensive diversity was revealed in CAR-T cells collected from peripheral blood over time post-infusion. Dynamic changes of CAR-T functional properties were observed at different time points. Of interest, this study revealed prognosis-associated dynamic changes of transcriptomic and functional properties of infused CAR-T cells in vivo, which will provide novel insights into the development of next-generation CAR-T cell strategies
Note:
Drs. Zongcheng Li and Lei Zhao contributed equally to this work.
Correspondence to Drs. Zongcheng Li, Bing Liu, Lilin Ye, Yu Lan and Liang Huang.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal